Livmarli clears severe skin growths in 2 boys with Alagille syndrome
Medication eased treatment-resistant itching and painful lesions
Written by |
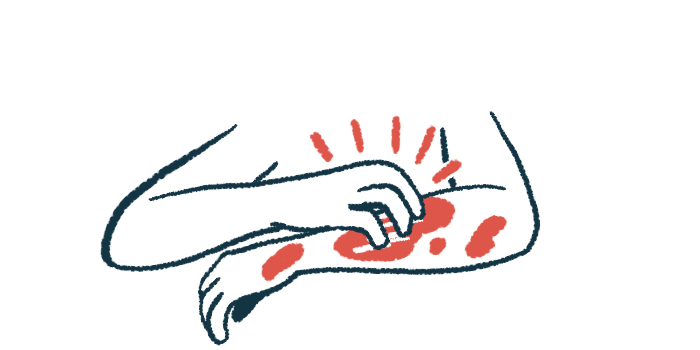
Livmarli (maralixibat) successfully cleared rare, debilitating fatty skin growths and eased chronic itching in two young boys with Alagille syndrome in the U.S., according to a new study.
The treatment resolved respiratory issues in one child and restored hand mobility in the other, marking the first time the medication has been documented to heal these specific, severe complications, researchers say.
The two unrelated patients suffered from treatment-resistant xanthomas, or waxy, cholesterol-rich lesions, that had failed to respond to standard therapies. In one rare case, the growths had spread to the boy’s airways, while the other boy’s widespread lesions were so severe they prevented him from using his hands properly.
“This is the first case report demonstrating airway [xanthomas] in ALGS [Alagille syndrome],” the researchers wrote. “The results reported herein suggest [Livmarli] may be a useful treatment option for patients with ALGS who have both pruritus [itching] and severe debilitating xanthomas.”
The cases were detailed in the study titled “Maralixibat for the treatment of severe xanthomas in two children with Alagille syndrome: Case reports,” published in the Journal of Pediatric Gastroenterology and Nutrition.
The role of bile and the Notch signaling pathway
A genetic disease, Alagille is caused primarily by mutations in the JAG1 gene and, less frequently, in the NOTCH2 gene. Both of these genes are part of the Notch signaling pathway, which is involved in embryonic development.
One of the most common defects in people with Alagille is a reduced number of bile ducts, which are the tubes that transport bile from the liver to the intestines. Bile is a fluid that helps in the breakdown and absorption of dietary fats and fat-soluble vitamins.
A reduced number of bile ducts slows or stops bile flow, a condition called cholestasis, leading to bile accumulation in the liver and leakage into the bloodstream. This can result in symptoms such as itchy skin and xanthomas.
Xanthomas, which have been reported in 24%-42% of Alagille patients, are thought to result from impaired bile acid flow from the liver. This can lead to higher-than-normal levels of cholesterol, a fatty molecule, in the blood, which promotes the formation of cholesterol-rich skin lesions.
Livmarli is an oral therapy approved to reduce itching in patients with Alagille syndrome. It works by blocking bile recycling from the intestines back to the liver, allowing more bile to exit the body in stool and reducing its accumulation in the liver and blood.
In the report, researchers describe the cases of two boys with Alagille in whom Livmarli helped to ease not only hard-to-treat itch, but also severe, debilitating xanthomas.
Both boys were diagnosed with Alagille in early infancy, at 1 and 3 months of age. Genetic testing identified a JAG1 mutation in the first boy, while no JAG1 or NOTCH2 mutations were detected in the second boy.
Both had fewer bile ducts and associated cholestasis. They were initially treated with ursodeoxycholic acid (UDCA, sold as Urso and Actigall) to help boost bile flow, fat-soluble vitamins, and a specialized formula for infants with fat malabsorption.
Itch and widespread xanthomas began between 6 and 9 months in the first boy, and at 2 years in the second boy, affecting his hands, and he also showed high cholesterol levels in the blood. Several itch medications were tried in both boys, with only mild symptomatic relief.
Xanthomas progressively worsened at the age of 1.5 years in the first boy, spreading to the airways and causing a croupy cough when he was about 2 years old. At the age of 10 years, the second boy experienced a worsening of xanthomas, particularly in his hands, affecting his fine motor skills.
Dramatic recovery and restored manual function
They started treatment with Livmarli at a dose of 190 micrograms per kilogram of body weight (mcg/kg) once daily, which was later increased to 380 mcg/kg per day. At the start of treatment, both boys had high blood levels of cholesterol and bile acids, the main component of bile.
The first boy experienced a clinically meaningful reduction in itch after six months of treatment, and his airway xanthomas were significantly flatter after 1.5 years. At the last follow-up, after 2.5 years of treatment, the xanthomas resolved, and he experienced minimal itch.
One year after starting Livmarli, the second boy also experienced resolution of xanthomas and clinically meaningful reductions in itch. He “regained manual function,” the team wrote, but the skin in his hands was thicker.
Both boys also had a significant reduction in bile acid and cholesterol levels.
“This is the first case report demonstrating the effectiveness and safety of [Livmarli] in patients with [Alagille with itching] and severe, debilitating xanthomas,” the researchers wrote.